Atomic Number And Mass Number
Key notes :
Definition of an Atom:
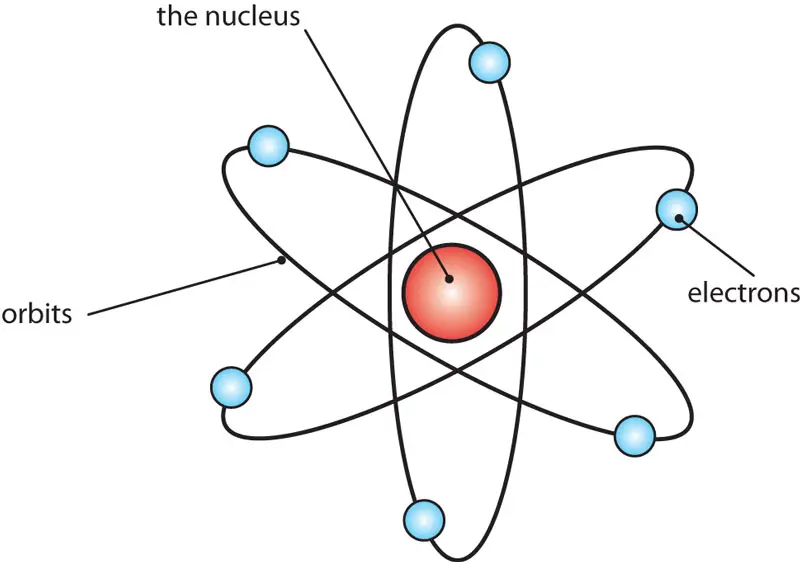
- An atom is the smallest unit of an element that retains the chemical properties of that element.
- It is made up of subatomic particles: protons, neutrons, and electrons.
Atomic Number (Z):
- The atomic number is defined as the number of protons in the nucleus of an atom.
- It determines the element’s identity and its position on the periodic table.
- For a neutral atom, the atomic number also equals the number of electrons.
- Example: Hydrogen has an atomic number of 1 (one proton), and carbon has an atomic number of 6 (six protons).
Mass Number (A):
- The mass number is the total number of protons and neutrons in the nucleus of an atom.
- It is calculated as: Mass Number (A) = Number of Protons (Z) + Number of Neutrons (N).
- Electrons have negligible mass, so they are not included in the mass number.
- Example: An oxygen atom with 8 protons and 8 neutrons has a mass number of 16.
Notation:
- Atoms are often represented using the notation: Element Symbol A Z
where A is the mass number and Z is the atomic number.
- For example, carbon can be written as 12 6.
Isotopes:
- Isotopes are atoms of the same element that have the same atomic number but different mass numbers due to varying numbers of neutrons.
- Example: Carbon-12 (12C) and Carbon-14 (14C) are isotopes of carbon with different numbers of neutrons.
Importance of Atomic Number:
- The atomic number determines the chemical properties of an element and its place in the periodic table.
- Elements are arranged in order of increasing atomic number.
Calculation Examples:
- If an atom has 11 protons and 12 neutrons:
- Atomic Number (Z) = 11
- Mass Number (A) = 11 + 12 = 23
- This atom would be represented as 23 11 Na
Let’s practice!

