How Are Electrons Distributed In Different Orbits?
Key Notes:
Structure of the Atom:

- Atoms consist of a central nucleus surrounded by electrons.
- The electrons revolve around the nucleus in specific paths called orbits or shells.
Energy Levels (Shells):
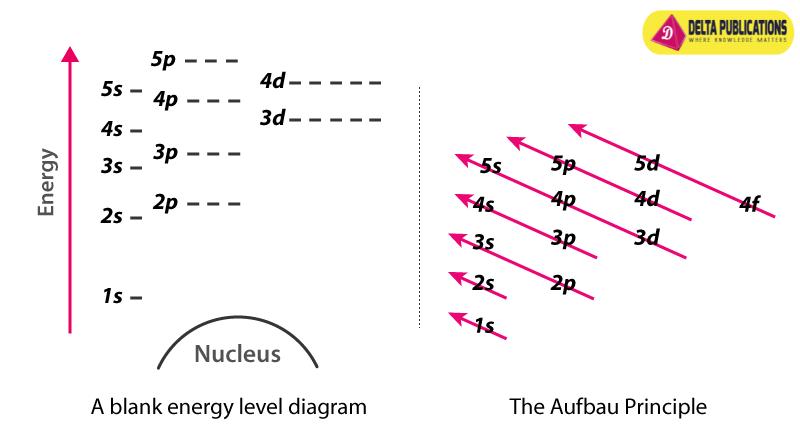
- Electrons occupy fixed energy levels around the nucleus, designated as K, L, M, N, etc.
- These energy levels are numbered as n = 1, 2, 3, 4, and so on.
Bohr’s Model of the Atom:
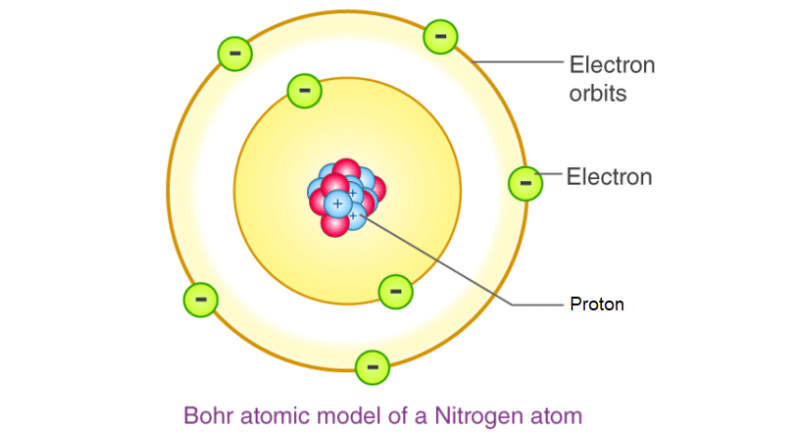
- Proposed by Niels Bohr, it suggests that electrons move in specific orbits with fixed energy.
- Electrons in lower orbits have less energy, while those in higher orbits have more energy.
Maximum Number of Electrons in a Shell:
- The number of electrons each shell can hold is determined by the formula 2n², where ‘n’ is the orbit number.
- K shell (n=1): 2 electrons
- L shell (n=2): 8 electrons
- M shell (n=3): 18 electrons
- N shell (n=4): 32 electrons
Filling of Electrons in Shells:
- Electrons fill the shells in order of increasing energy (from K to N).
- Lower energy levels are filled first before moving to higher levels.
Octet Rule:
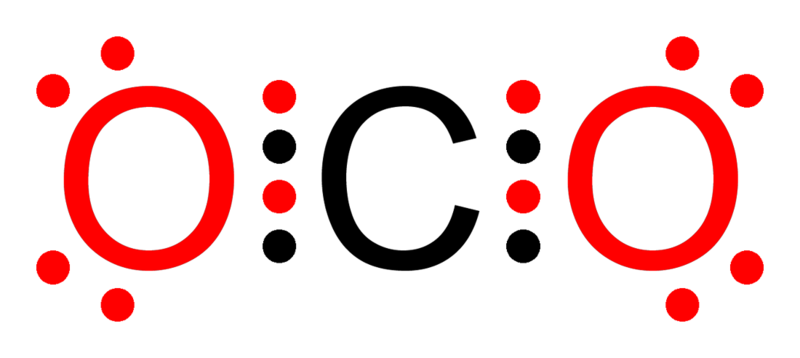
- Atoms tend to fill their outermost shell to achieve a stable configuration with 8 electrons, similar to noble gases.
- Elements with less than 8 electrons in their outer shell are reactive.
Valence Electrons:
- Electrons in the outermost shell are called valence electrons.
- These determine the chemical properties of an element.
Example of Electron Distribution:
- Carbon (Atomic number = 6): 2 electrons in K shell, 4 electrons in L shell (2, 4)
- Sodium (Atomic number = 11): 2 electrons in K shell, 8 in L shell, 1 in M shell (2, 8, 1)
Stability and Reactivity:
- Atoms are most stable when their outermost shell is completely filled.
- Atoms with incomplete outer shells will gain, lose, or share electrons to achieve stability.
Let’s practice!

