Structure Of An Atom
key notes :
Introduction to the Atom:
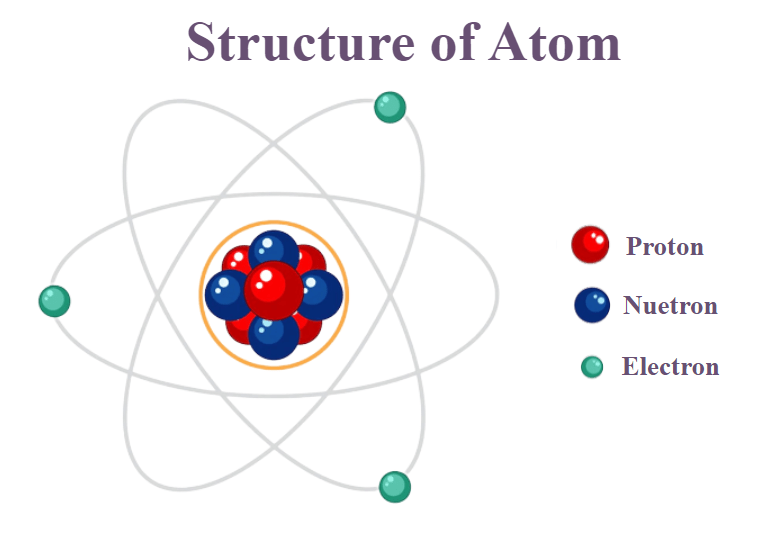
- An atom is the smallest unit of an element that retains the chemical properties of that element.
- Atoms are the basic building blocks of matter.
Subatomic Particles:
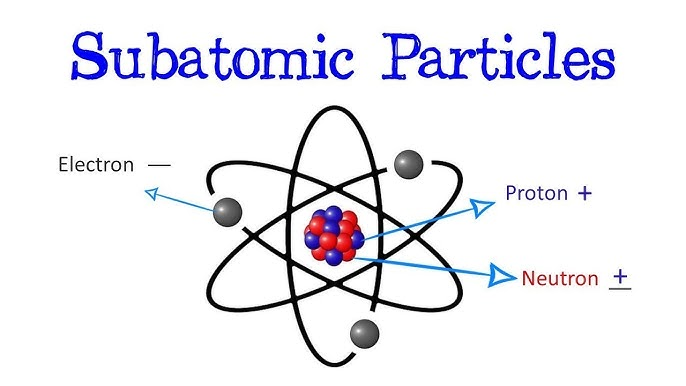
- Protons: Positively charged particles located in the nucleus.
- Neutrons: Neutral particles (no charge) also found in the nucleus.
- Electrons: Negatively charged particles that revolve around the nucleus in orbitals.
Atomic Structure:
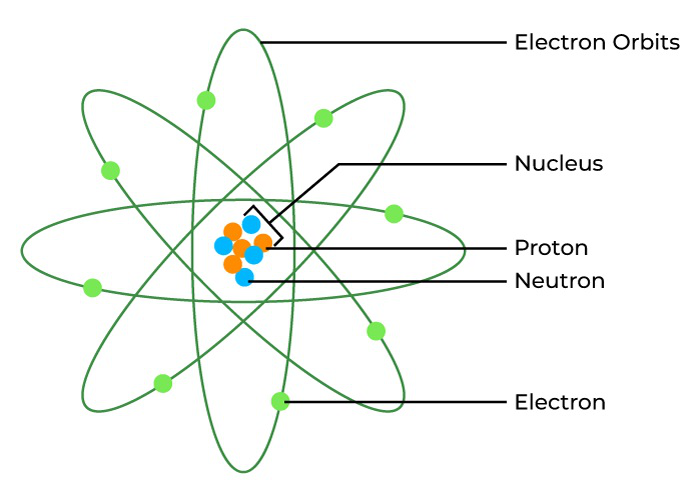
- The nucleus, located at the center of the atom, contains protons and neutrons.
- Electrons orbit the nucleus in energy levels or shells.
- Atoms are mostly empty space, with the nucleus occupying only a tiny part of the atom’s volume.
Atomic Number (Z):
- Represents the number of protons in the nucleus.
- Determines the identity of an element (e.g., hydrogen has an atomic number of 1).
Mass Number (A):
- The sum of the number of protons and neutrons in an atom’s nucleus.
- Mass Number = Number of Protons + Number of Neutrons.
Isotopes:
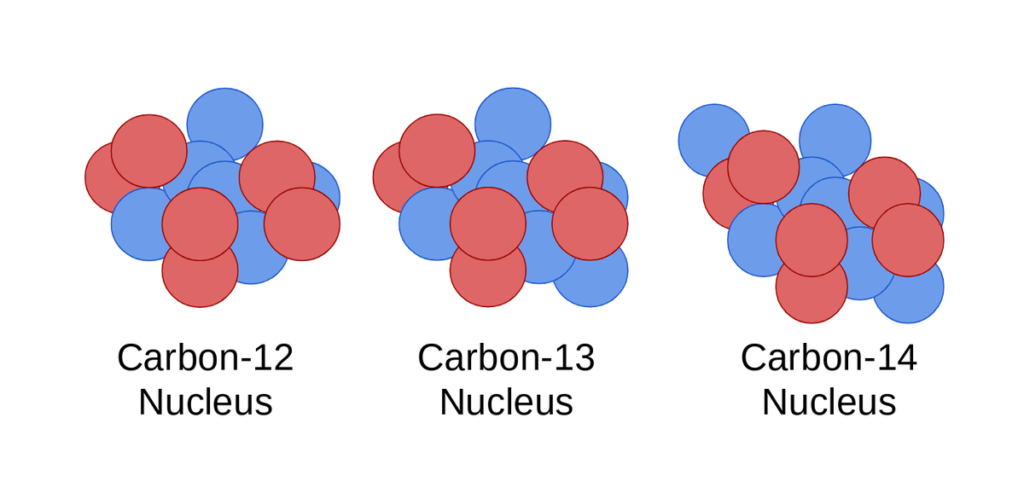
- Atoms of the same element with the same atomic number but different mass numbers (due to varying numbers of neutrons).
- Example: Carbon has isotopes like Carbon-12, Carbon-13, and Carbon-14.
Atomic Models:
- Thomson’s Model: Described the atom as a sphere of positive charge with embedded electrons (like a “plum pudding”).
- Rutherford’s Model: Proposed that atoms have a small, dense nucleus with electrons orbiting around it.
- Bohr’s Model: Suggested that electrons move in fixed orbits (shells) around the nucleus and can jump between energy levels.
Electron Configuration:
- Electrons are arranged in shells around the nucleus according to their energy levels.
- The maximum number of electrons in a shell is given by the formula 2n22n^22n2, where nnn is the shell number (e.g., 1st shell can hold 2 electrons, 2nd shell can hold 8 electrons, etc.).
Valence Electrons:
- Electrons in the outermost shell of an atom.
- Determine the chemical reactivity and bonding behavior of an element.
Octet Rule:
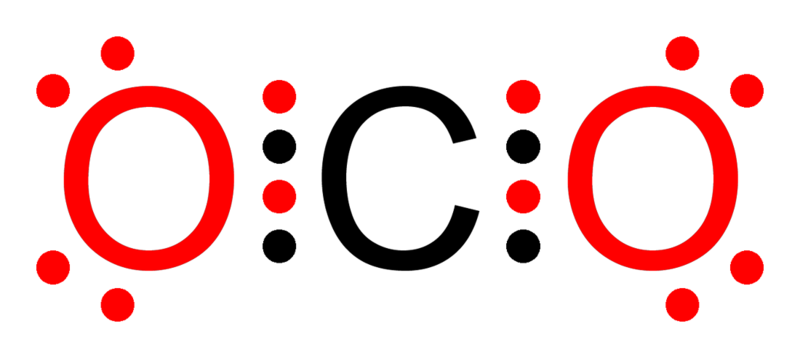
- Atoms tend to gain, lose, or share electrons to achieve a stable configuration with 8 electrons in their outermost shell.
Ions:
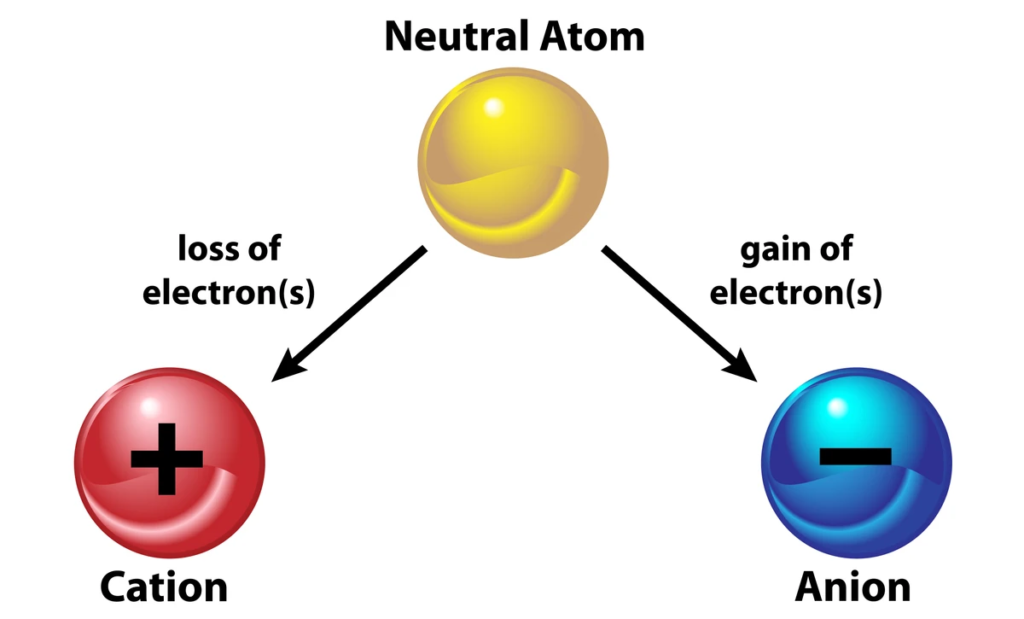
- Atoms that have gained or lost electrons.
- Cations: Positively charged ions (loss of electrons).
- Anions: Negatively charged ions (gain of electrons).
Atomic Theory Development:
- The understanding of the atomic structure evolved over time with contributions from scientists like Dalton, Thomson, Rutherford, and Bohr.
Let’s practice!

