Writing Chemical Formulae
Key Notes:
Definition of Chemical Formula:
- A chemical formula represents the elements in a compound and their relative proportions.
- It consists of symbols for elements and subscripts indicating the number of atoms of each element in the molecule.
Symbols of Elements:
- Every element has a unique symbol (e.g., H for hydrogen, O for oxygen, Na for sodium).
- These symbols are used in writing chemical formulas.
Types of Chemical Formulas:
- Empirical Formula: Represents the simplest whole-number ratio of elements in a compound (e.g., CH for benzene).
- Molecular Formula: Represents the actual number of atoms of each element in a molecule (e.g., C6H6 for benzene).
- Structural Formula: Shows the arrangement of atoms in a molecule.
Writing Formulae for Ionic Compounds:
- Ionic compounds are formed by the transfer of electrons between metals and non-metals.
- To write the formula:
- Write the symbol of the metal (cation) first.
- Write the symbol of the non-metal (anion) next.
- Balance the charges to ensure the compound is neutral (e.g., NaCl, MgO).
- Use subscripts to indicate the number of ions needed to balance the charges (e.g., Al₂O₃ for aluminum oxide).
Writing Formulae for Covalent Compounds:
- Covalent compounds are formed by the sharing of electrons between non-metals.
- Use prefixes (mono-, di-, tri-, etc.) to indicate the number of atoms of each element (e.g., CO₂ for carbon dioxide, N₂O₄ for dinitrogen tetroxide).
- Do not use prefixes for the first element if it has only one atom (e.g., CO for carbon monoxide).
Polyatomic Ions:
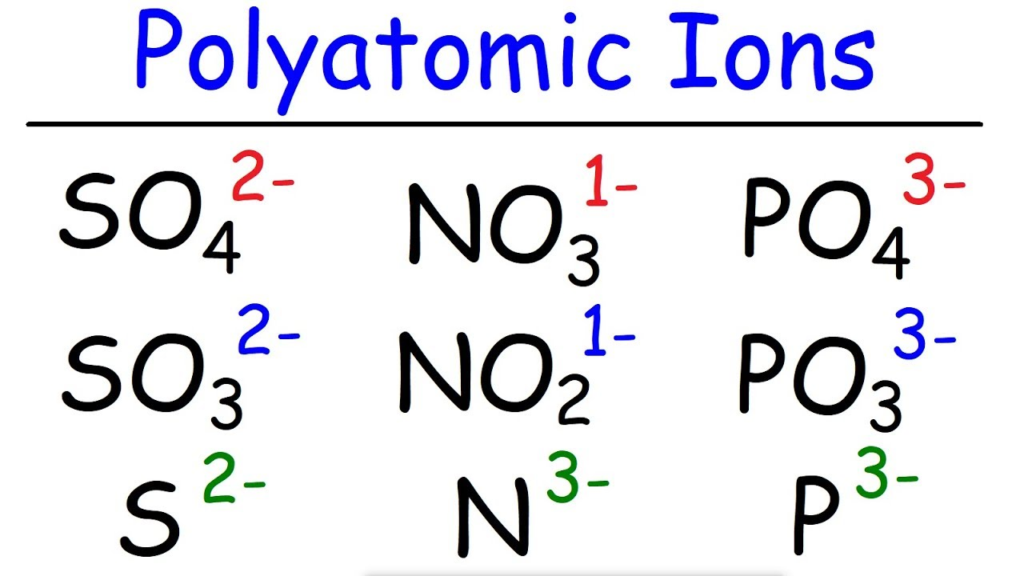
- Some compounds contain polyatomic ions (groups of atoms that carry a charge), such as sulfate (SO₄²⁻), nitrate (NO₃⁻), and ammonium (NH₄⁺).
- In these cases, the polyatomic ion is treated as a single unit and placed in parentheses if more than one is needed (e.g., Ca(NO₃)₂ for calcium nitrate).
Balancing the Charges:
- The total positive charge must equal the total negative charge to ensure the compound is neutral.
- The number of cations and anions can be adjusted by using appropriate subscripts.
Common Examples:
- Sodium chloride: NaCl (1:1 ratio of Na+ and Cl-).
- Calcium carbonate: CaCO₃ (Ca²⁺ and CO₃²⁻ in a 1:1 ratio).
- Water: H₂O (two hydrogen atoms for every oxygen atom).
Rules for Writing Chemical Formulae:
- For Ionic Compounds: Balance charges, write the cation first, and use the lowest ratio of ions.
- For Covalent Compounds: Use prefixes to indicate the number of atoms of each element.
Let’s practice!

