What Is A Molecule?
Key Notes:
Definition of a Molecule:

- A molecule is a group of two or more atoms bonded together. These atoms can be of the same or different elements.
- Molecules are the smallest unit of a chemical compound that can exist independently while retaining the chemical properties of that compound.
Types of Molecules:
- Diatomic molecules: Composed of two atoms, usually of the same element (e.g., oxygen (O₂), nitrogen (N₂)).
- Polyatomic molecules: Composed of three or more atoms, which may be the same or different elements (e.g., water (H₂O), carbon dioxide (CO₂)).
Atoms in Molecules:
- Molecules are made up of atoms that are held together by chemical bonds, which can be covalent, ionic, or metallic bonds.
- Covalent bonds: Formed when two atoms share electrons.
- Ionic bonds: Formed when one atom gives up electrons to another atom, creating positive and negative ions that attract each other.
Molecular Formula:
- A molecular formula represents the number and types of atoms in a molecule (e.g., H₂O for water, CO₂ for carbon dioxide).
- It does not show the arrangement of atoms, which would require a structural formula.
Importance of Molecules:
- Molecules make up all matter, including solids, liquids, and gases.
- Biological molecules like proteins, DNA, and carbohydrates are essential for life processes.
- Chemical reactions involve the breaking and forming of bonds between molecules.
Molecule vs Atom:
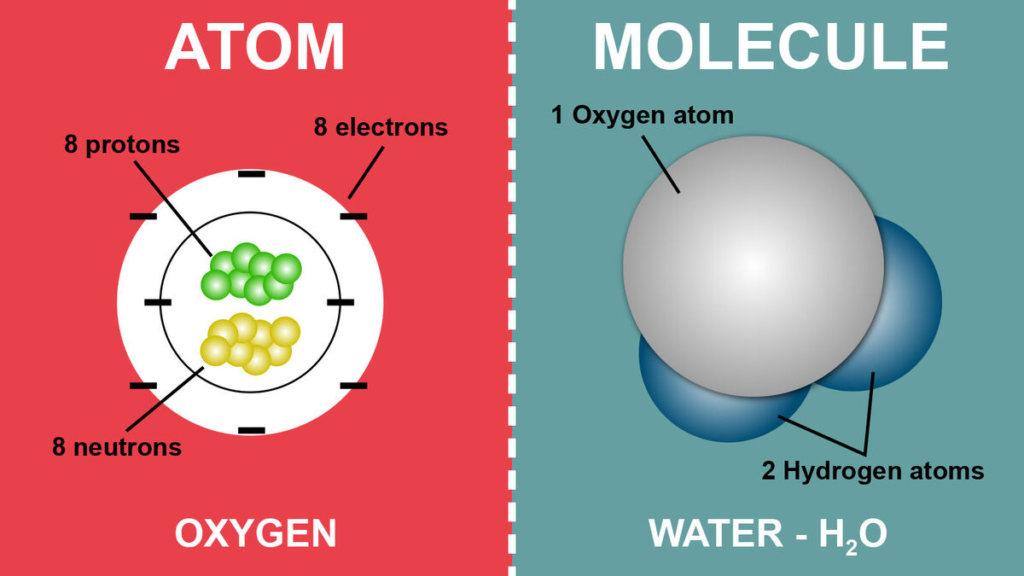
- An atom is the smallest unit of an element, while a molecule is a combination of atoms.
- Atoms can exist independently (e.g., noble gases like helium, neon), but molecules need at least two atoms to exist.
Properties of Molecules:
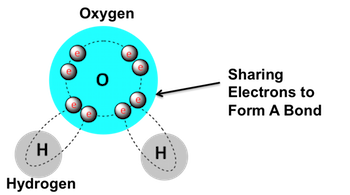
- The properties of a molecule (such as its size, shape, and polarity) depend on the types of atoms involved and how they are bonded together.
- For example, water molecules are polar, which means they have a slight electrical charge difference, allowing them to form hydrogen bonds.
Examples of Molecules:
- Water (H₂O): Composed of two hydrogen atoms and one oxygen atom.
- Carbon dioxide (CO₂): Composed of one carbon atom and two oxygen atoms.
- Oxygen (O₂): Composed of two oxygen atoms.
- Methane (CH₄): Composed of one carbon atom and four hydrogen atoms.
Let’s practice!

