What Is An Atom?
what is an atom by Delta publications
Key Notes :-
Definition of an Atom:
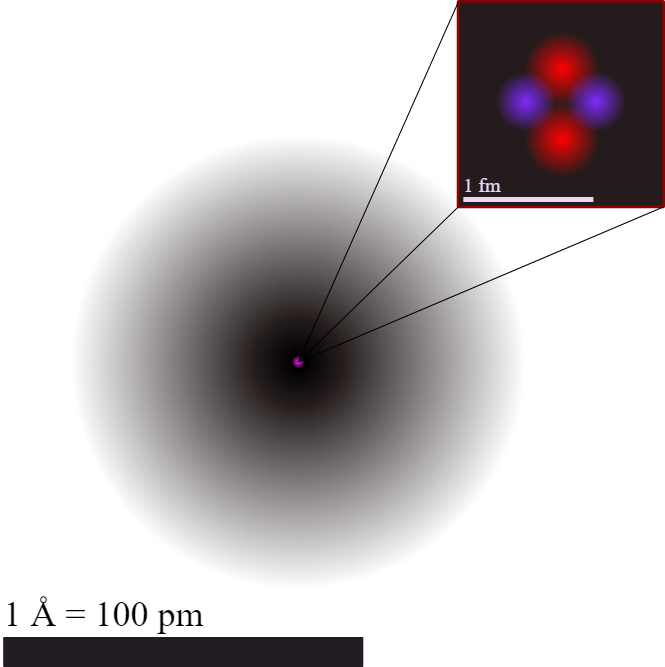
- An atom is the smallest unit of matter that retains the properties of an element. It cannot be broken down into simpler substances by chemical means.
Structure of an Atom:
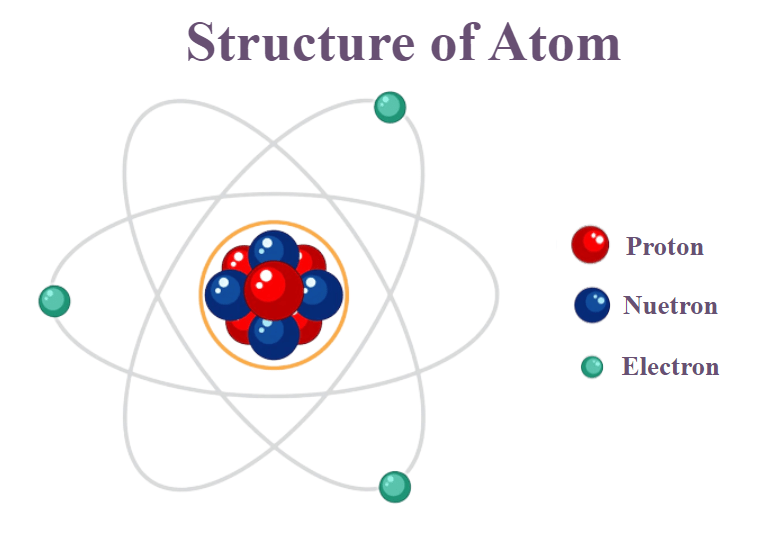
- Atoms consist of three fundamental particles: protons, neutrons, and electrons.
- Protons (positively charged) and neutrons (neutral) are located in the nucleus, which is the dense central part of the atom.
- Electrons (negatively charged) orbit the nucleus in regions called electron shells or energy levels.
Size of an Atom:

- Atoms are extremely small, with diameters typically around 0.1 to 0.5 nanometers (1 nanometer = 10−9).
- The nucleus is even smaller but contains nearly all of the atom’s mass.
Atomic Number and Mass Number:
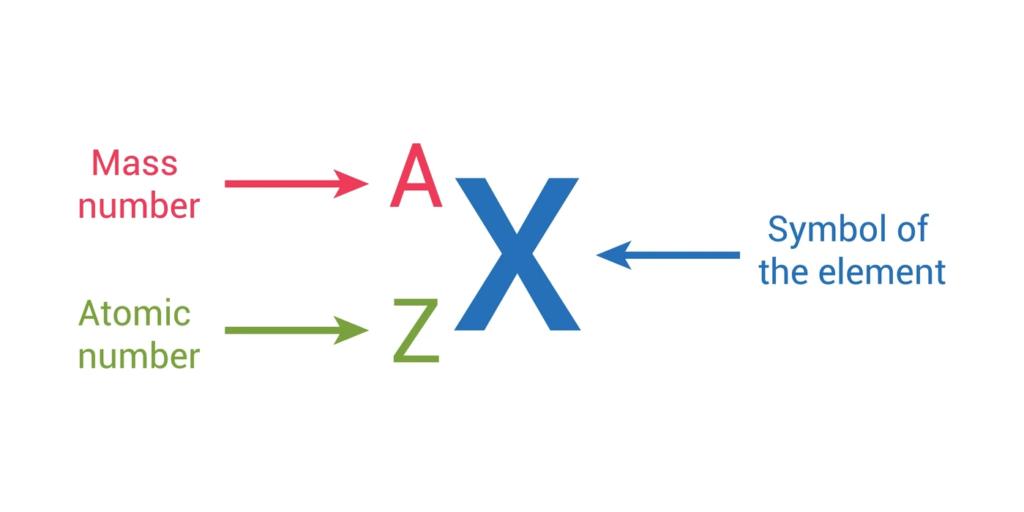
- The atomic number represents the number of protons in an atom and determines the element.
- The mass number is the total number of protons and neutrons in the nucleus.
- Atoms of the same element can have different numbers of neutrons; these are called isotopes.
Electrons and Energy Levels:
- Electrons are arranged in energy levels (shells) around the nucleus.
- The first energy level can hold up to 2 electrons, the second up to 8 electrons, and so on.
- The arrangement of electrons determines an atom’s chemical properties.
Neutral Atoms vs. Ions:
- In a neutral atom, the number of protons equals the number of electrons.
- If an atom gains or loses electrons, it becomes an ion. A positively charged ion is a cation, and a negatively charged ion is an anion.
Discovery of the Atom:
- The concept of the atom was first proposed by Democritus, a Greek philosopher, around 400 BCE.
- The modern understanding of atomic structure was developed through experiments by scientists like John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr.
Importance of Atoms:
- Atoms are the building blocks of all matter. Everything around us, from the air we breathe to the materials we use, is composed of atoms.
- Understanding atomic structure is fundamental to chemistry and physics, influencing fields like medicine, electronics, and materials science.
Let’s practice!

