Matter Changes Its State
Key notes :
Definition of Matter States:
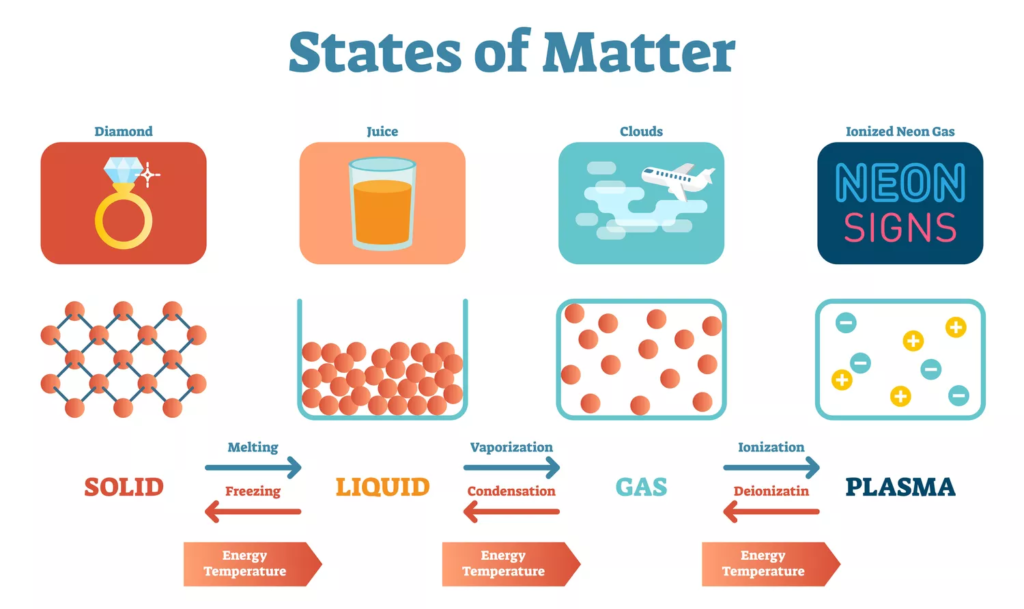
- Matter exists in three primary states: solid, liquid, and gas.
- The state depends on the arrangement, movement, and energy of particles.
Interconversion of States:
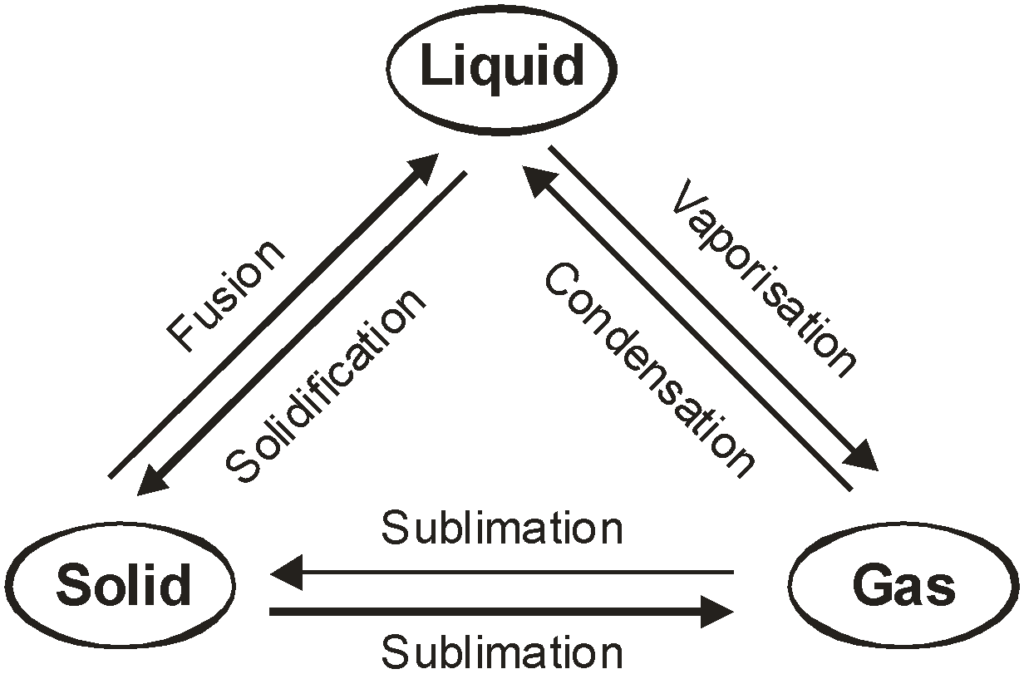
- Matter can change states (e.g., solid to liquid, liquid to gas) due to changes in temperature or pressure.
- These changes are physical changes, as they don’t alter the chemical composition.
Processes of State Changes:
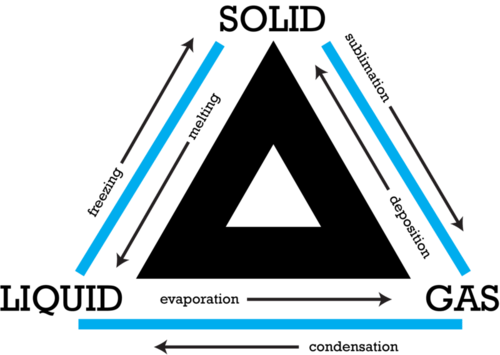
- Melting: Solid → Liquid (e.g., ice melting into water).
- Freezing: Liquid → Solid (e.g., water freezing into ice).
- Evaporation: Liquid → Gas (e.g., water turning into vapor at its surface).
- Boiling: Liquid → Gas (e.g., water boiling at 100°C).
- Condensation: Gas → Liquid (e.g., water vapor condensing into droplets).
- Sublimation: Solid → Gas directly (e.g., dry ice sublimating into carbon dioxide gas).
Role of Temperature:
- Adding heat increases the kinetic energy of particles, causing them to overcome intermolecular forces and change state.
- Removing heat decreases kinetic energy, allowing particles to come closer and change state.
Latent Heat:
- Latent Heat of Fusion: The heat required to change 1 kg of a solid into a liquid at its melting point.
- Latent Heat of Vaporization: The heat required to change 1 kg of liquid into gas at its boiling point.
Effect of Pressure:
- Increasing pressure can compress particles and force matter into a denser state (e.g., gas to liquid).
- Decreasing pressure allows particles to expand and move apart (e.g., liquid to gas).
Examples in Daily Life:
- Boiling water for cooking.
- Ice cubes melting in a drink.
- Fog formation from condensation.
- Sublimation of mothballs or dry ice.
Energy and State Changes:
- State changes are energy-dependent:
- Endothermic Processes: Absorb energy (e.g., melting, evaporation).
- Exothermic Processes: Release energy (e.g., freezing, condensation).
Heating Curve of Water:
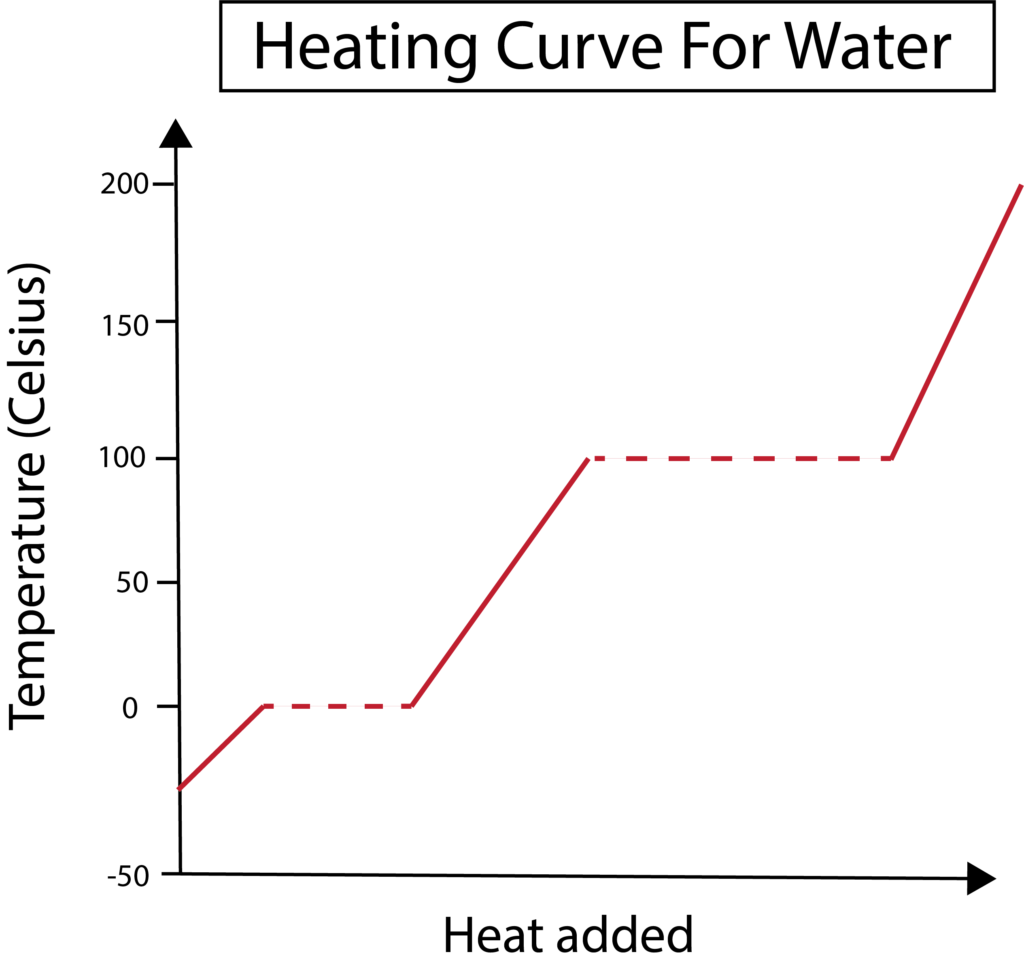
- A graph showing temperature changes as heat is added:
- Flat portions represent phase changes (e.g., melting and boiling).
- Sloped portions indicate temperature increase in a single state.
Applications:
- Refrigeration systems use condensation and evaporation cycles.
- Industrial processes like distillation involve state changes.
Let’s practice!

