Characteristics Of Particles Of Matter
Key Notes:
Matter is Made Up of Particles
- All matter is composed of tiny particles that are not visible to the naked eye.
Particles are Very Small
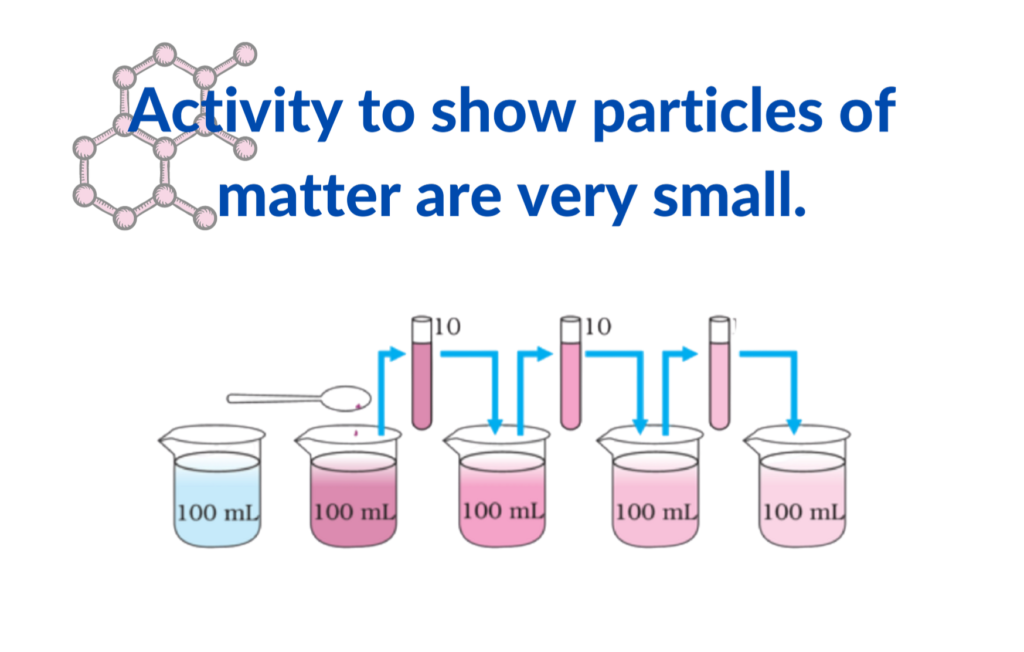
- The size of particles is extremely small and can only be observed under advanced equipment or indirectly through phenomena like diffusion.
Particles Have Spaces Between Them
- Particles are not tightly packed and have spaces between them.
- The amount of space varies in solids, liquids, and gases:
- Solids: Minimal spaces.
- Liquids: Moderate spaces.
- Gases: Maximum spaces.
Particles are in Constant Motion

- Particles of matter are always moving, showcasing kinetic energy.
- The motion increases with temperature:
- Higher temperatures → More energy → Faster motion.
Particles Attract Each Other
- Particles have forces of attraction between them.
- Strength of attraction varies:
- Solids: Strongest attraction.
- Liquids: Moderate attraction.
- Gases: Weakest attraction.
Diffusion and Mixing
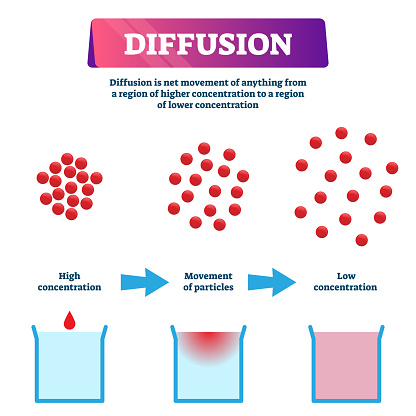
- The motion and spaces between particles allow for diffusion (mixing of particles).
- Example: Gases like oxygen and carbon dioxide mix easily in the atmosphere.
Compression and Expansion
- Gases can be compressed significantly due to large spaces between particles, while solids and liquids are less compressible.
Impact of Temperature on Particles
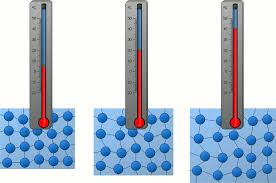
- Temperature affects particle movement:
- Increase in temperature → Increased speed and energy.
- Decrease in temperature → Reduced motion.
Let’s practice!

