Matter In Our Surroundings
Key Notes:
Definition of Matter
- Matter is anything that has mass and occupies space.
- It can be perceived through the senses.
Physical Nature of Matter
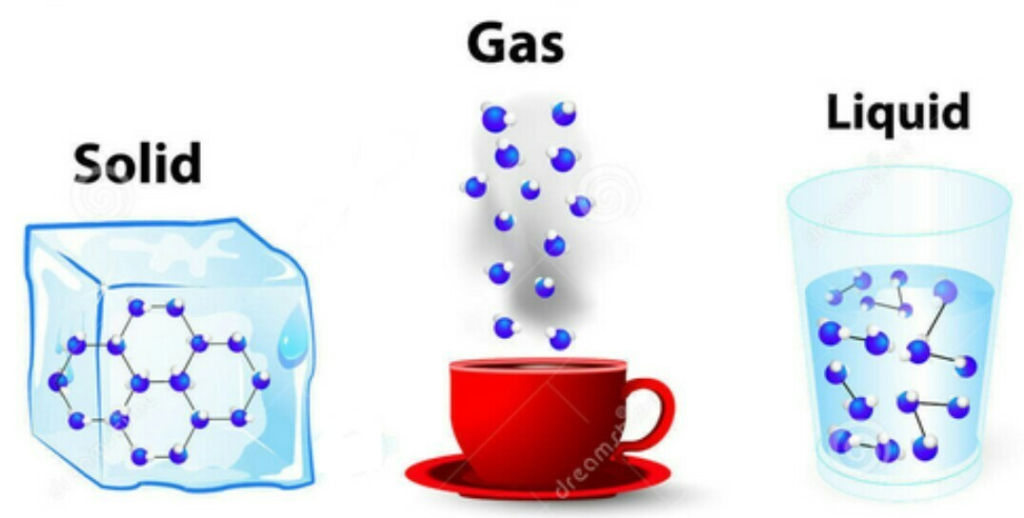
- Matter is made up of tiny particles.
- These particles are too small to be seen with the naked eye.
Characteristics of Particles of Matter

- Particles are continuously moving: They possess kinetic energy and move faster at higher temperatures.
- Particles have space between them: The space determines the state of matter (solid, liquid, or gas).
- Particles attract each other: The force of attraction varies among solids, liquids, and gases.
States of Matter
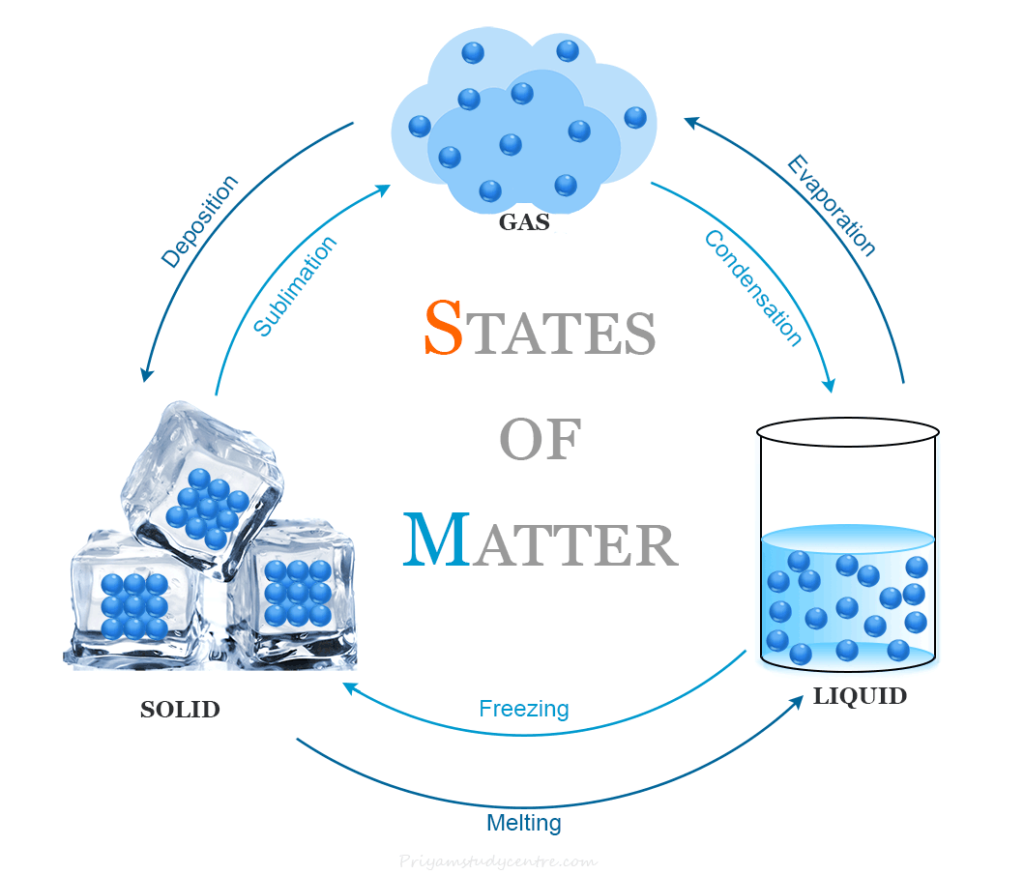
- Solids: Fixed shape and volume, strong intermolecular force, minimal particle movement.
- Liquids: Fixed volume, take the shape of the container, weaker intermolecular force compared to solids, particles can slide past each other.
- Gases: No fixed shape or volume, very weak intermolecular force, particles move freely and quickly.
Changes in States of Matter

- Effect of Temperature:
- Increasing temperature increases the kinetic energy of particles, causing changes in state (solid to liquid to gas).
- Melting point: Temperature at which a solid turns into a liquid.
- Boiling point: Temperature at which a liquid turns into gas.
- Sublimation: Some solids directly change into gas without becoming liquid (e.g., camphor, iodine).
- Effect of Pressure:
- Increasing pressure can change a gas to a liquid by bringing particles closer.
Latent Heat
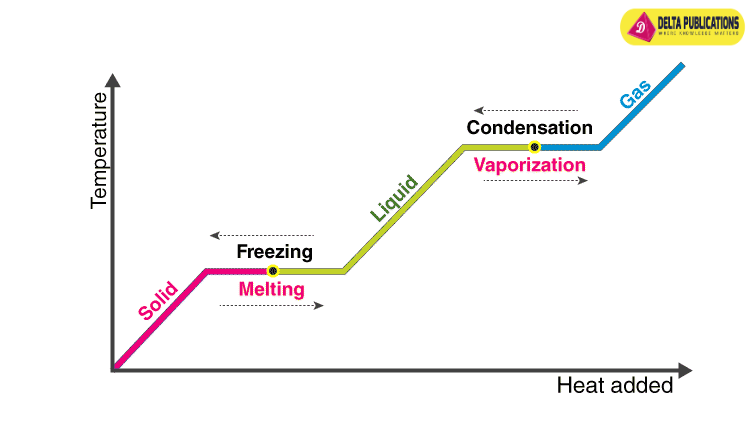
- Latent heat of fusion: Heat required to change 1 kg of a solid into a liquid without changing temperature.
- Latent heat of vaporization: Heat required to change 1 kg of a liquid into gas without changing temperature.
Evaporation
- Process of liquid changing into vapor at a temperature below its boiling point.
- Factors affecting evaporation:
- Surface area: More area leads to faster evaporation.
- Temperature: Higher temperature increases evaporation rate.
- Humidity: Higher humidity decreases evaporation rate.
- Wind speed: Faster wind increases evaporation.
Cooling Effect of Evaporation
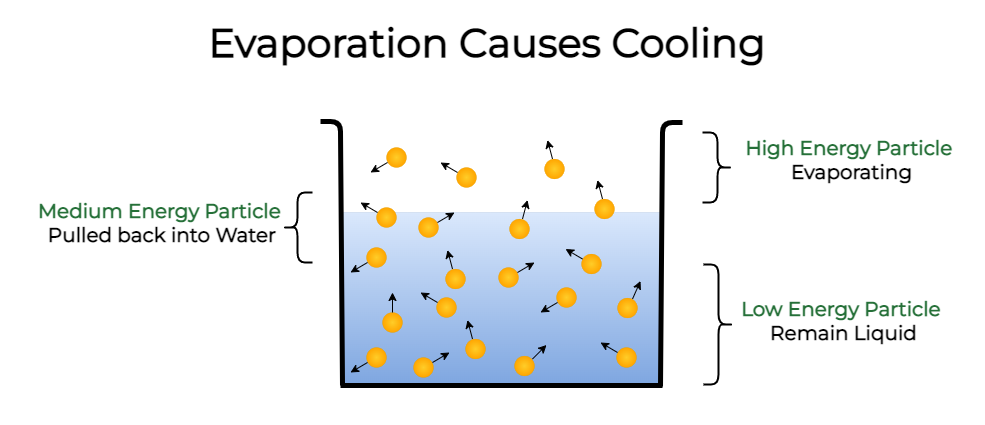
- During evaporation, particles absorb energy from their surroundings, leading to a cooling effect (e.g., perspiration cools the body).
Let’s practice!

